Do you find yourself flipping through pages of notes just to find a particular chemical reaction? Well, you don’t have to do that anymore! Here are all the important chemical reactions Year 12 students must know!
Table of contents:
- Module 5: Equilibrium and Acid Reactions
- Module 6: Acid/Base Reactions
- Module 7: Organic Chemistry
- Additional Reactions
Module 5: Equilibrium and Acid Reactions
Combustion of metals
Word equation: metal + oxygen → metal oxide
Examples:
Burning magnesium: \( 2Mg_{(s)} \ + \ O_{2(g)} \ → \ 2M_{g}O_{(s)} \)
Burning steel wool: \( 4Fe_{(s)} \ + \ 3O_{2(g)} \ → \ 2Fe_{2}O_{3(s)} \)
Combustion of hydrocarbons and alcohols
1. Complete combustion produces carbon dioxide and water.
Examples:
Hydrocarbon: \( C_{3}H_{8(g)} \ + \ 5O_{2(g)} \ → \ 3CO_{2(g)} \ + \ 4H_{2}O_{(l)} \)
Alcohol: \( C_{2}H_{5}OH_{(l)} \ + \ 3O_{2(g)} \ → \ 2CO_{2(g)} \ + \ 3H_{2}O_{(l)} \)
2. Incomplete combustion occurs when insufficient oxygen is present for a complete reaction to occur.
Water and carbon monoxide (\(CO_{(g)}\)) and/or soot (\(C_{(s)}\)) are produced in an incomplete combustion.
Photosynthesis
Word equation: carbon dioxide + water → glucose + oxygen
Chemical equation: \( 6CO_{2(g)} \ + \ 6H_{2}O_{(l)} \ → \ C_{6}H_{12}O_{6(s)} \ + \ 6O_{2(g)} \)
Cobalt chloride hydrate equilibrium
\( [CoCl_{4}]^{2-} \ _{(aq)} \ (blue) \ + \ 6H_{2}O_{(l)} \ ⇌ \ [Co(H_{2}O)_{6}]^{2+} \ _{(aq)} \ (pink) \ + \ 4Cl^{-} \ _{(aq)} \)
Iron thiocyanate equilibrium
\( Fe^{3+} \ _{(aq)} \ \text{(pale yellow)} \ + \ SCN^{-} \ _{(aq)} \ ⇌ \ [FeSCN]^{2+} \ _{(aq)} \ \text{(deep red)} \)
Nitrogen dioxide equilibrium
\( 2NO_{2(g)} \ (brown) \ ⇌ \ N_{2}O_{4(g)} \ \text{(colourless)} \)
Dissolution equilibrium
A saturated solution of an ionic compound will be in dynamic equilibrium.
Example: \( CuSO_{4(aq)} \ ⇌ \ Cu^{2+} \ _(aq) \ + \ SO_{4^{2-} (aq) }\)
Precipitation reactions
A good knowledge of the solubility rules is essential to determining whether a precipitation reaction will occur when solutions containing ionic compounds are mixed.
Example:
Full formula equation: \( BaCl_{2(aq)} \ + \ Na_{2}SO_{4(aq)} \ → \ BaSO_{4(s)} \ + \ 2NaCl_{(aq)} \)
Net ionic equation: \( Ba^{2+} \ _{(aq)} \ + \ SO_{4^{2-} (aq)} \ → \ BaSO_{4(s)} \)
Module 6: Acid/Base Reactions
Ionisation
1. Strong acids undergo complete ionisation in water
Example: \( HCl_{(aq)} \ + \ H_{2}O_{(l)} \ → \ H_{3}O^{+} \ _{(aq)} \ + \ Cl^{-} \ _{(aq)} \)
\( (HCl_{(aq)} \ → \ H^{+} \ _{(aq)} \ + \ Cl^{-} \ _{(aq)}) \)
2. Weak acids undergo partial ionisation in water
Example: \( CH_{3}COOH_{(aq)} \ + \ H_{2}O_{(l)} \ ⇌ \ H_{3}O^{+} \ _{(aq)} \ + \ CH_{3}COO^{-} \ _{(aq)} \)
\( (CH_{3}COOH_{(aq)} \ ⇌ \ H^{+} \ _{(aq)} \ + \ CH_{3}COO^{-} \ _{(aq)}) \)
3. Strong bases undergo complete dissociation in water
Example: \( NaOH_{(aq)} \ → \ Na^{+} \ _{(aq)} \ + \ OH^{-} \ _{(aq)} \)
4. Weak bases undergo partial ionisation in water
Example: \( NH_{3(aq)} \ + \ H_{2}O_{(l)} \ ⇌ \ NH_{4^{+} (aq)} \ + \ OH^{-} \ _{(aq)} \)
Neutralisation
Word equation: acid + base → salt + water
Example: \( H_{2} \color{red}{SO_{4}} _{(aq)} \ + \ 2 \color{blue}{K} OH_{(aq)} \ → \ \color{blue}{K_{2}} \color{red}{SO_{4}} _{(aq)} \ + \ H_{2}O_{(l)} \)
Acids and carbonates
Word equation: acid + carbonate → salt + water + carbon dioxide
Example: \( 2H \color{red}{NO_{3}} _{(aq)} \ + \ \color{blue}{Na} _{2} CO_{3(s)} \ → \ 2 \color{blue}{Na} \color{red}{NO_{3}} _{(aq)} \ + \ H_{2}O_{(l)} \ + \ CO_{2(g)} \)
Acids and metals
Word equation: acid + metal → salt + hydrogen gas
Example: \( 2H \color{red}{Cl} _{(aq)} \ + \ \color{blue}{Mg} _{(s)} \ → \ \color{blue}{Mg} \color{red}{Cl} _{2(aq)} \ + \ H_{2(g)} \)
Acid and ammonia:
Word equation: acid + ammonia → ammonium salt
Example: \( HCl_{(aq)} \ + \ NH_{3(aq)} \ → \ NH_{4}Cl_{(aq)} \)
Module 7: Organic Chemistry
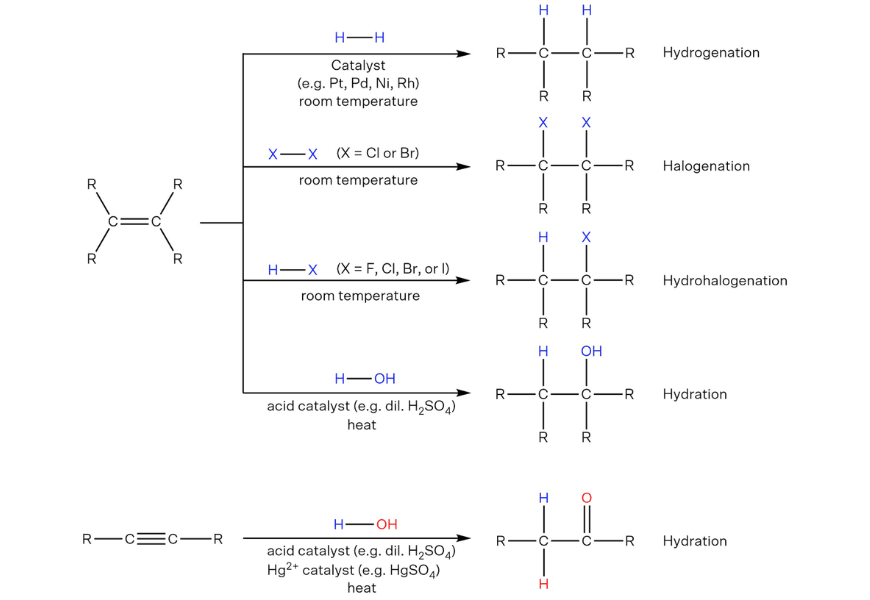
Addition reactions of unsaturated hydrocarbons
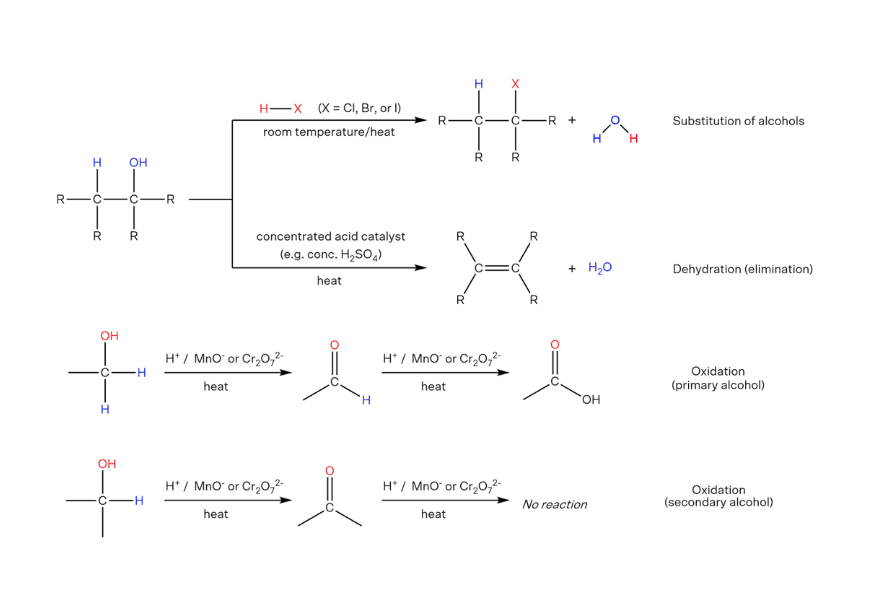
Reactions of alcohols
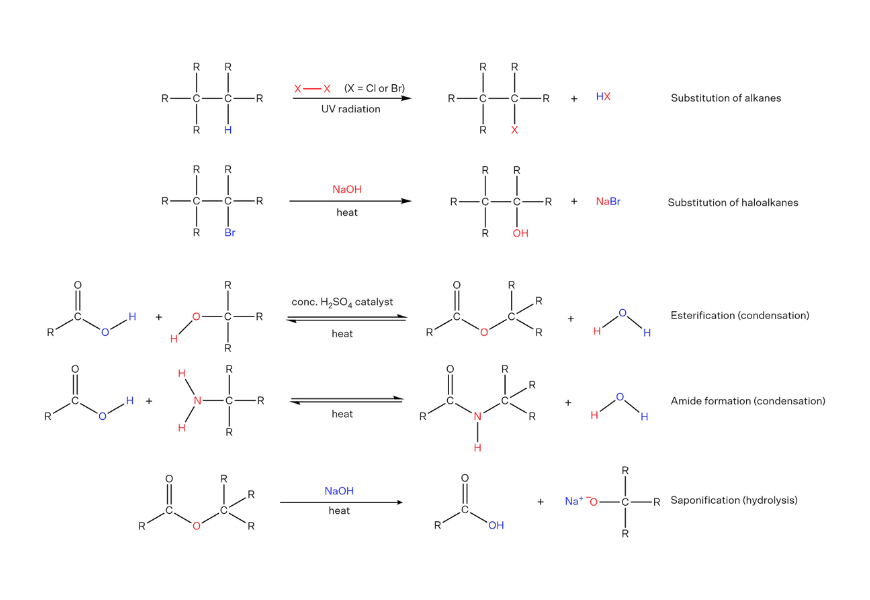
Additional organic reactions
Combustion of hydrocarbons and alcohols
1. Complete combustion produces carbon dioxide and water.
Examples:
Hydrocarbon: \( C_{3}H_{8(g)} \ + \ 5O_{2(g)} \ → \ 3CO_{2(g)} \ + \ 4H_{2}O_{(l)} \)
Alcohol: \( C_{2}H_{5}OH_{(l)} \ + \ 3O_{2(g)} \ → \ 2CO_{2(g)} \ + \ 3H_{2}O_{(l)} \)
2. Incomplete combustion occurs when insufficient oxygen is present for a complete reaction to occur.
Water and carbon monoxide \((CO_{(g)})\) and/or soot \((C_{(s)})\) are produced in an incomplete combustion.
Fermentation
Fermentation of glucose: \( C_{6}H_{12}O_{6(aq)} \ → \ 2C_{2}H_{5}OH_{(l)} \ + \ 2CO_{2(g)} \)
Conditions for fermentation:
- Zymase (enzyme in yeast)
- Aqueous solution of sugar
- Anaerobic condition
- Typically 30–40 °C, but depends on yeast strain
Additional reactions that are good to know
Redox
1. Oxidation: \( M_{(s)} \ → \ M^{+} \ _{(aq)} \ + \ e^{-} \)
2. Reduction: \( M^{+} \ _{(aq)} \ + \ e^{-} \ → \ M_{(s)} \)
3. Redox equation: Combine oxidation and reduction equations and balance electrons.
Example
- Oxidation: \( Cu(s) → Cu2+(aq) + 2e- \)
- Reduction: \( Ag+(aq) + e- → Ag(s) \)
- \(1:2 \) for \( Cu:Ag \), so combine with 2x reduction equation
- \( Cu_{(s)} \ + \ 2Ag^{+} \ _{(aq)} \ + \ 2e^{-} \ → \ Cu^{2+} \ _{(aq)} \ + \ 2e^{-} \ + \ 2Ag_{(s)} \)
- Cancel the electrons: \( Cu_{(s)} \ + \ 2Ag^{+} _{(aq)} \ → \ Cu^{2+} \ _{(aq)} \ + \ 2Ag_{(s)} \)
4. Limewater test: \( Ca(OH)_{2(aq)} \ + \ CO_{2(g)} \ → \ CaCO_{3(s)} \ + \ H_{2}O_{(l)} \)
,
