Here are eight HSC Chemistry Exam Tips written by Matrix Chemistry Teacher Louise Donnelly.
1. Know What You’re Being Assessed On.
It is important to recognise the HSC Chemistry examination will assess a multitude of skills – it is not just about ‘what you know.’
The HSC exam tests your ability to:
- relate chemical concepts;
- interpret questions & analyse data;
- successfully manage your time; and
- communicate effectively.
Developing your examination technique – answering the question, eliminating careless errors, using correct terminology – is essential for success in the HSC.
2. Know Your Exam Structure.
Knowing the structure of any examination enables you to practice similar questions, pre-allocate your time and have all necessary equipment.
General Instructions
• Reading time – 5 minutes
• Working time – 3 hours
• Write in blue or black pen
• Draw diagrams using a pencil
• Calculators approved by NESA may be used
• A formula sheet, data sheet and a Periodic Table are provided at the back of this paper.
Total marks – 100
SECTION I Pages 2-1
• Attempt Questions 1-20
• Allow about 35 minutes for this section
SECTION II Pages 13-32
• Attempt Questions 21-34
• Allow about 2 hours and 25 minutes for this section
You have 3 hours for 100 marks. This equates to 1.8 minutes per mark. This means you should spend around 10 minutes on a 5 mark question. The use of this time is essential – especially for written response questions. Since you will be required to answer around 20 written response questions (ranging from 1-7 marks), losing 1 or 2 marks per question equates to losing 20 – 40%. However, if you can gain that extra 1 or 2 marks, your improvement is substantial.
3. Know How to Approach Questions.
The key to gaining the maximum mark for a question is to THINK before you respond. Use your allocated time to analyse the question, plan your response carefully, and then work within that framework to produce a clear, logical and concise response. Look at the question. The answer space provided and the marks allocated are guides to the maximum length of response required. Similarly, the key word used in the question gives an indication of the depth of the required response. You may plan to use dot points, diagrams and/or tables; this will help avoid internal contradictions. For example, a question on the use of plastics could include a table that relates structure to a property and to a use. If the question requires a judgement (eg. justify, evaluate,) consider the use of sub-headings such as ‘advantages’, ‘disadvantages’ and ‘assessment’. It is essential to address the question being asked. Many students identify the key verb but neglect to follow through with further analysis. What specifically are you asked to explain or evaluate? What terms should be defined? Are there any specific requirements such as a labelled diagram or balanced equation? Underlining all keywords will focus your attention on the exact task required.
EXAMPLE: 2007 HSC CHEMISTRY
Question 19 (7 marks)
There are many benefits and problems associated with the use of radioisotopes in industry and medicine.
Evaluate the impact on society of the use of radioisotopes in both industry and medicine. In your answer, give examples of specific radioisotopes, making reference to their chemical properties.
Highlighting these “keywords” will help ensure all requirements are met. In the above example you must make a judgement (based on criteria) on the impact on society. You should also recognise the term radioisotope needs to be defined and you must discuss their use in both industry AND medicine. You must also provide more than one example and provide chemical information on each.
See Also: Glossary of Key Words
4. Know How to Answer Questions.
Whatever the type of question you are answering it is essential to pay attention to detail.
Communicate with Words
Your aim is to provide a concise and relevant answer and display good chemistry.
The key is to be SPECIFIC
• do not repeat the question as part of the response.
• identify a specific impact rather than offer a general statement, such as the chemicals “harm” the environment or the substance is “dangerous”
• use specific terminology associated with the course this includes the terminology associated with equipment e.g. dissolved in a volumetric flask and then filled up to the calibration line.
• correct chemical equations should also be included to illustrate the chemical processes described. – ensure all formulas are correct and equations balanced. – remember to check all charges are balanced when writing ionic equations.
RE-READ the question and your response to ensure you have met all requirements and that it “reads well.” The following sample demonstrates a coherent and logical response.
EXAMPLE: 2006 Chemistry HSC
Question 24 (4 marks)
Early in the twentieth century, Fritz Haber developed a method for preparing ammonia.
(b) Evaluate the significance of Haber’s discovery at that time in world history.
Early in the 20th century there was need for an industrially synthesized fertilizer to feed the world’s growing population. Also the growing militancy in Germany needed a product for explosives. Haber’s discovery was able to meet these demands. The method also contributed to Germany’s effort in WWI as it insulated Germany from the cutting off of the import saltpeter (the current natural fertiliser) from South America and allowed explosives to be made from nitric acid. Therefore Haber’s discovery had a significant impact on Germany in the early 20th century.
5. Know How to Communicate With Numbers.
When answering questions involving calculations in the HSC you need to set out your work clearly, showing all steps in your working, and expressing your answers with the correct number of significant figures.
If you are required to substitute values into a formula, for example calculating pH, you must state the formula clearly. You should then show working with the values substituted into the equation, before performing the calculation.
The data you use should be accurately transcribed from The periodic table and data sheet for calculations. Atomic mass of carbon? 12.01 It is advisable to use all 4 figures in your calculation, even if you have to round down for your final answer
Use the correct number of significant figures for your final answer. Remember rounding-off needs to be done at the end of the calculation.
When setting out your working for numerical questions think carefully about the units to be used and the quantities to be substituted into formulae. That is, you need to be “unit-conscious”. You must be consistently alert to the possibility that you may have to convert mL to L, or kg to g.
Familiarise yourself with commonly used prefixes. Converting micrograms (µg) to milligrams (mg) is not achievable without such knowledge.
Similarly, concentration can be expressed in different ways – mol/L, ppm, %w/v. Identifying that 1 ppm is equivalent to 1 mg/L is essential in many analytical/environmental questions.
6. Know How to Communicate Visually.
When drawing structural formulae, make sure that all covalent bonds are shown between atoms.
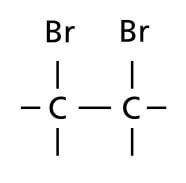 |
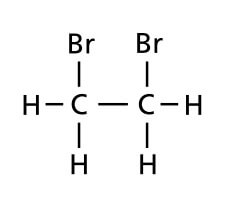 |
||
| Do not draw | When you mean | ||
The covalent bond between O and H in the hydroxyl group of alkanols should be shown.
When drawing a polymer, show two or more monomer units joined together (rather than the simple abbreviated structure) and the bonds at each end of the chain open (without the use of ‘n’ molecules).
Use appropriate equipment, for example, pencils and a ruler to draw diagrams and graphs.
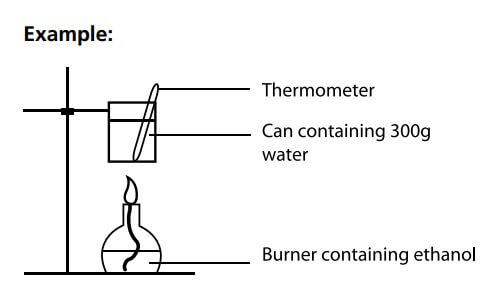
7. Know How to Draw Equipment Correctly.
Drawing scientifically is a fundamental skill that requires attention to detail. It is not appropriate to “sketch” the equipment. Your diagram should be to scale, ruled and clearly labelled. As specifically stated in the HSC Chemistry General Instructions:
• Draw diagrams using pencil
Example:
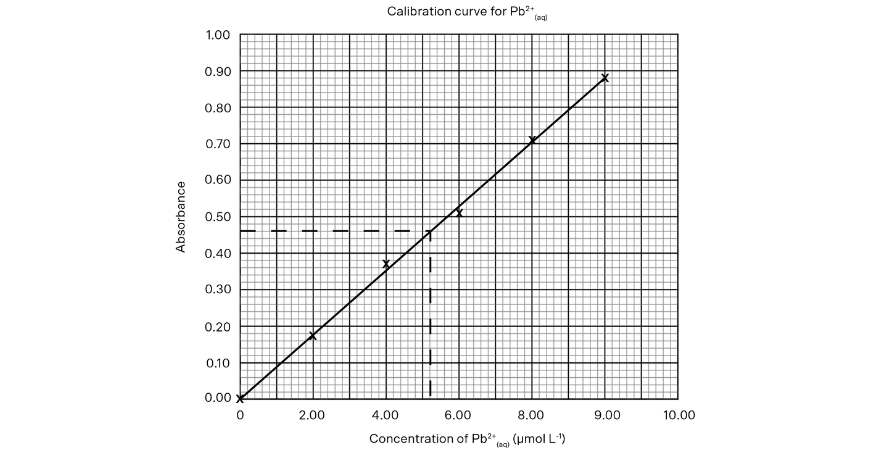
8. Know How to Draw Graphs Correctly.
It is quite certain that you will have to deal with a graph in one way or another in the HSC examination. A few hints to remember:
• Plot the points accurately by marking the point with a cross and use a pencil and ruler to draw the line of best fit.
• Include scales that are linear and clearly label both axes (with units if applicable).
• Ensure you use the majority of the grid provided.
• Extrapolation should be avoided since predictions are often invalid.
If a value is obtained by an interpolated estimate, clearly show on the graph how the value was obtained. A clear plastic ruler helps to plot points that are further from the axes and rule straight lines of best fit.