Acing HSC Chemistry involves mastering both the conceptual understandings demanded of you from the course and being able to deliver on the often extensive amounts of details for these concepts.
As such, your study approach should involve a balance of understanding and direct learning of each topic.
In this article, we discuss these 4 study hacks to ace Chemistry,
- Create summary notes
- Create a chemical equation list
- Attempt past paper questions
- Prep response scaffolds
Chemistry hack 1: Create summary notes
There is no doubt that the HSC Chemistry course is dense content-wise. Make concise study notes consistently so you grasp the content quickly and thoroughly. This is the most effective hack.
Step 1: Compile resources to use for your notes
The first step for this process is to compile the resources you will be using for your notes.
It is important that you rely on resources from several different sources.
This prevents you from missing out on any interesting or important details which make up concepts, as well as expose yourself to different explanations of the same concept.
These resources may include various HSC Chemistry textbooks (two to three is an ideal amount), school notes, online videos and websites.
Step 2: Read and summarise relevant chapters that you covered in school
Once you have compiled the relevant resources, take the time to read the relevant chapters or sections of the resource for the Chemistry concept you just covered at school.
Take the time to summarise the relevant information from each resource on a single A4 page.
Step 3: Further summarise and condense into dot points
The next step is to then further summarise and condense each of the A4 pages into a series of dot points for your personal notes.
Structuring your Chemistry notes is particularly important – though a much more time consuming process, a good approach to note taking in Chemistry is to hand write the notes.
This makes diagram drawing and equation writing much more efficient than when using digital tools.
It also allows you to adopt a much more pragmatic mindset to note taking – less is more.
Organise your notes under the relevant dot point and module so that when it comes to revision, you begin to familiarise yourself with the details and concepts relevant to each of the question types.
Colour code your notes so that Chemistry definitions are written in one colour, diagrams and equations drawn in another, and any other interesting facts can be annotated in another.
Step 4: Update Chemistry notes at the end of each week
You should aim to update your Chemistry notes at the end of each school week.
In this way, you are consistently revising chemical concepts, and gives you time in the lead up to exams to complete past paper questions and rely on your notes for quick review of specific concepts you may be struggling with.

Image: Prinica’s colour co-ordinated Chemistry notes
Chemistry hack 2: Chemical equation list
While the HSC Chemistry course has a relevant data sheet with equations and values that you should be relatively familiar with, a great way to improve the note-taking process is to allocate space at the start of your handwritten notes for chemical formulas.
Often, the more difficult calculation or experimental based questions in the Chemistry trial and HSC papers are questions which require you to deduce the products of a reaction given the reactants.
In order to improve your ability to deduce the reaction occurring in these scenarios, make your own formula sheet which summarises and lists some common chemical reactions which fall under each category.
For example, reactions between an acid and base versus an acid and carbonate.
Be sure to include important details such as the effect of having a concentrated versus dilute acid on particular chemical reactions.

Image: Kenvin’s chemical equation notes

Image: Martina’s Chemistry equation notes
Chemistry hack 3: Past paper questions
In order to truly ace HSC Chemistry, it is important to practise applying the concepts you have learned to unseen questions.
For Chemistry, in particular, practicing responding to exam questions and marking your responses strictly against the suggested solutions is important as most of the concepts are relatively connected to one another.
It is often difficult to discern which concepts are relevant to the question.
So, in order to risk including unnecessary information and omitting other points which are awarded marks, you must begin to familiarise yourself with the typical key words in questions and the syllabus dot points that these key words are related to.
A good strategy for improving is to focus on the two question types of the HSC Chemistry paper – multiple choice questions and short answer questions.
1. Multiple choice
The multiple choice questions in Chemistry are often testing your ability to make several calculations without error and deduce the best response from a list of relatively close answers.
One way to improve your performance in the multiple choice section is to compile all of the MCQ from past HSC papers and complete them in a single sitting, taking short breaks in between.
After each paper mark the MCQ and record the kinds of mistakes you are making.
As you repeat this process, you will notice that your performance will improve as you begin to pin point the distractors much more quickly.
2. Short answer
The short answer questions demand that you be able to quickly pin point which parts of a specific module are important to include in your response.
One way to improve your performance in the short answer section is to list what details you would include for a given response in dot point form, rather than spend time writing out the response in its entirety.
Compare the list of dot points you would include with the sample solution and this will quickly allow you to refine your exam technique as you begin to see the particular details you tend to omit or unnecessarily include.

Image: Areebah’s Chemistry marked past paper
Chemistry hack 4: Compile response scaffolds
There are some question types which when drawn from the HSC Chemistry syllabus can be changed very little across papers.
For the typical longer response question types, it is a good idea to have a model answer prepared in dot point form using your notes.
There is no need to memorise this response, but instead, have the scaffold prepared such that if that question type to appear in an upcoming exam, you can quickly formulate a response which you can be fairly certain will be awarded all of the marks.
How do we do this? Let’s see:
Remember, exam questions will always be based on syllabus dot points. So, it is worthwhile going through the syllabus to identify dot points that are open-ended or require detailed explanations.
For instance, consider this dot point in Module 7 under Hydrocarbons:
- construct models, identify the functional group, and write structural and molecular formulae for homologous series of organic chemical compounds, up to C8: – alkanes – alkenes – alkynes
This dot point only requires students to identify and recall the various groups and terminology for organic compounds. It is highly unlikely that there will be a long response question based on this straight-forward dot point.
On the other hand, another dot point in Module 7 could be framed in a long response question, as it offers the opportunity for the student to discuss and apply higher-level content:
- conduct an investigation to compare the properties of organic chemical compounds within a homologous series, and explain these differences in terms of bonding
This question from Chemistry HSC 2020 is an example of a long response based on this dot point.
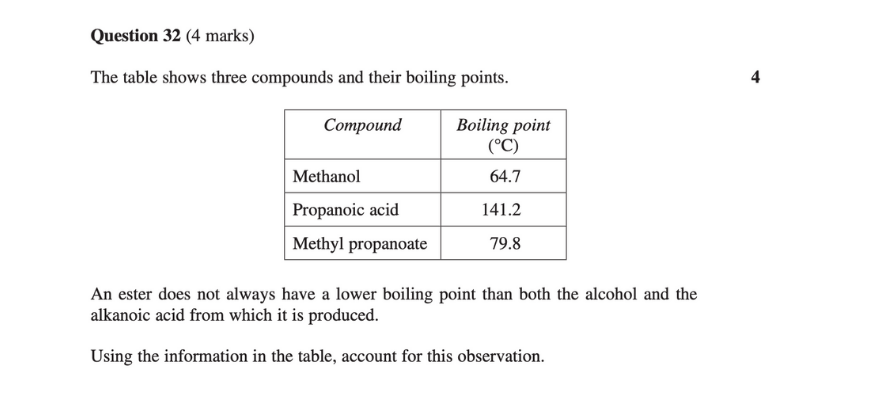
Here’s an example of a scaffold that would help you prepare for such questions based on this dot point:
- Boiling points of compounds are determined by intermolecular forces between their molecules.
- Alcohol and carboxylic acid both have hydrogen bonding (the strongest type of intermolecular force).
- Since carboxylic acid displays more hydrogen bonding, it has stronger intermolecular forces than alcohols with the same/similar number of carbons.
- Esters do not display hydrogen bonding at all, and hence generally have weaker intermolecular forces than alcohols and carboxylic acid.
- However, esters with long carbon chains and high molecular masses will have relatively strong dispersion forces, which may result in an ester having stronger intermolecular forces than an alcohol or acid.
- In the case of the above question, the ester (methyl propanoate) has a significantly greater molecular mass and hence stronger dispersion forces compared to alcohol (methanol).
- The ester’s dispersion forces result in its overall intermolecular force being stronger than that of the alcohol, despite the alcohol’s hydrogen bonding.
- As a result, methyl propanoate requires more thermal energy to overcome its intermolecular bonds compared to alcohol, which means that it has a higher boiling point.
- However, propanoic acid still has a higher boiling point than methyl propanoate because it has a similar molecular mass to methyl propanoate and multiple hydrogen bonding donors and acceptors per molecule.
