These are the responses to the 2021 Chemistry paper, which might be discovered right here on the NESA web site.
Part 1: A number of Alternative Questions
| Query | Reply | Rationalization | ||||||||||||
| 1 | A | At dynamic equilibrium, the speed of the ahead and reverse response are equal. | ||||||||||||
| 2 | B | Silver nitrate produces the ions Ag+ and NO3–. Primarily based on the solubility guidelines, all nitrates are soluble (NO3–), so the precipitation response will contain Ag+. Of the out there ions, Ag+ will type a precipitate with Cl–. | ||||||||||||
| 3 | A | The compound proven is a secondary amide. The prefix for the compound title is given by the longest carbon chain (propan-) and the suffix is given by the purposeful group (-amide). The alkyl chain that’s sure to the amide nitrogen is handled as a substituent, and is known as by utilizing an uppercase, italicised N to point that the substituent is hooked up to the nitrogen atom (N-methyl). In consequence, the title of the compound is N-methylpropanamide. | ||||||||||||
| 4 | C | Esterification entails the response of a carboxylic acid and an alcohol to provide an ester. The ester is known as utilizing the scaffold alkyl alkanoate, the place the alkyl part of the compound originates from the alcohol, and the alkanoate part originates from the carboxylic acid. In consequence, ethyl pentanoate is synthesised by the response of ethanol and pentanoic acid. | ||||||||||||
| 5 | B | The tactic utilized by the scholar comprises two errors:
a. The coed rinses the burette with deionised water fairly than the titrant (sodium hydroxide). This is able to outcome within the titrant answer being diluted, which means {that a} bigger titre could be required to succeed in the top level. In consequence, the measured focus of acetic acid would seem larger than the precise focus. General, the calculated focus of acetic acid could be larger than the precise focus. |
||||||||||||
| 6 | C | Oka is simply affected by temperature, so will stay the identical after dilution. The extent of ionisation will increase as the answer is diluted. The rise within the extent of ionisation might be understood by first contemplating the equilibrium for the ionisation of a weak acid and the equilibrium fixed expression: HA(aq) ⇋ H+(aq) + A–(aq) Oksp = [H+][A–]/[HA] By diluting the answer, the concentrations of [H+], [A–], and [HA] will all lower. In consequence, the response quotient (Q) based mostly on these concentrations will probably be lower than the equilibrium fixed (Ok) (Q < Ok). Thus, the equilibrium will shift proper to provide extra ions and enhance the extent of ionisation. |
||||||||||||
| 7 | A | Bromomethane can have a decrease boiling level than methanol. Methanol has hydrogen bonding as its dominant intermolecular pressure, whereas bromomethane has dipole-dipole forces because the dominant intermolecular pressure. As dipole-dipole forces are weaker than hydrogen bonds, much less thermal power is required to beat these forces for the substance to boil. | ||||||||||||
| 8 | D | Cleaning soap molecules have two parts: a hydrophilic head group and a hydrophobic tail. The hydrophobic tail kinds stronger adhesive forces with the oil, whereas the hydrophilic head group kinds stronger adhesive forces with water. In consequence, the cleaning soap molecules orientate in order that the tail is contained inside the oil and the pinnacle group is on the interface of the oil and the water. | ||||||||||||
| 9 | D | The wavelength used within the measurement of an analyte by UV-Vis spectroscopy corresponds to the absorption most of the analyte. Consequently, the solvent used to arrange the pattern mustn’t have a powerful absorbance on this area in any other case it will impression on the measured focus of the analyte. | ||||||||||||
| 10 | B | The polymer chain proven is a kind of condensation polymer referred to as a polyester. These polymers are fashioned by the response of monomers containing each a carboxylic acid and a hydroxyl group, ensuing within the elimination of water (H2O). Primarily based on the connectivity proven within the polymer chain, the hydroxyl group would must be positioned on the carbon adjoining to the carboxylic acid group. | ||||||||||||
| 11 | C | The equilibrium fixed expression for this response is:
Ok = [Cl2] As this expression reveals, the equilibrium is impartial of the quantity of PCl5(s), and solely depends upon the focus of Cl2(g). In consequence, the addition of PCl5(s) is not going to disturb the equilibrium. |
||||||||||||
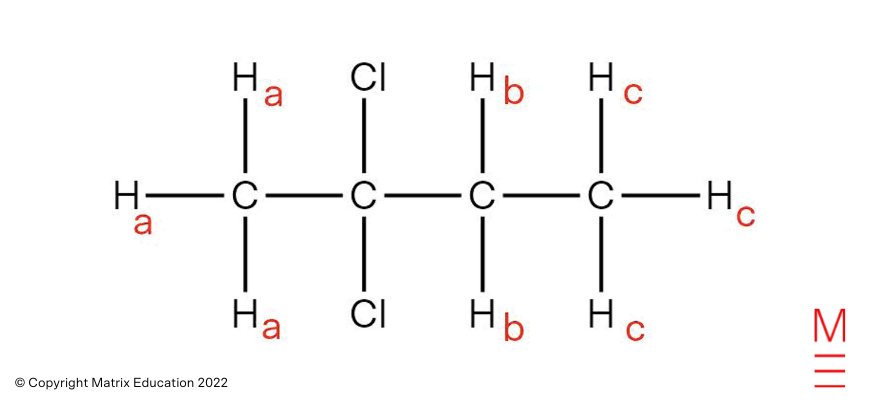
| 12 | A | The m/z peak at 98 within the mass spectrum corresponds to the molecular ion, and is in step with a molecular formulation of C6H10O. The carbon-13 NMR spectrum depicts 4 distinctive 13C environments, indicating that symmetry exists within the compound. | ||||||||||||
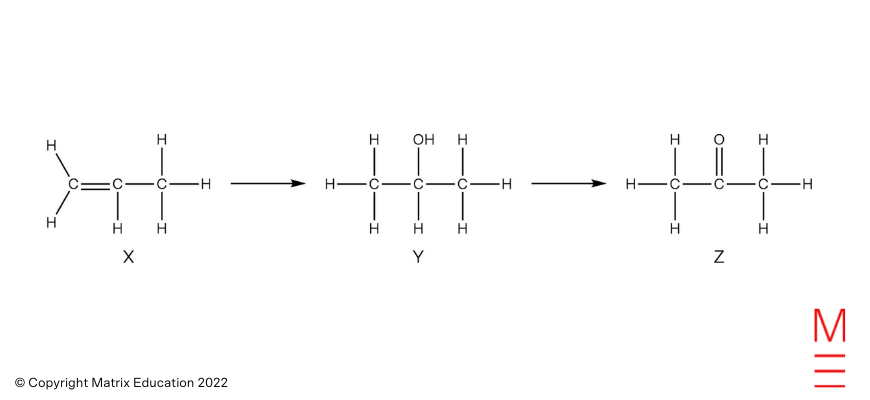
| 13 | C | Alkenes below hydration situations react to type alcohols. The oxidation of a secondary alcohol leads to the formation of a ketone.
|
||||||||||||
| 14 | D | The focus of the diluted nickel pattern is discovered utilizing the calibration curve, and is roughly 0.0021 mol L-1. The focus of the undiluted pattern is discovered by:
c(undiluted) = c(diluted) x V(diluted) / V(undiluted) = (0.0021 x 250) / 10 = 0.0529 mol L-1 n(Ni) = c x V = 0.0529 x 0.050 = 0.002645 mol m(Ni) = n x MM = 0.002645 x 58.69 = 0.16 g which is closest to 0.15 g. |
||||||||||||
| 15 | B | HCl(aq) + NaOH(aq) NaCl(aq) + H2O(aq)
n(HCl) = c x V = 0.20 x 0.020 = 0.0040 mol n(NaOH) = c x V = 0.50 x 0.020 = 0.010 mol Due to this fact, HCl is limiting. n(NaOH) remaining = 0.010 – 0.0040 = 0.0060 mol c(NaOH) = n / V = 0.0060 / 0.040 = 0.15 mol L-1 = [OH–] pOH = -log10[OH–] = -log10(0.15) = 0.8239 pH = 14 – pOH = 14 – 0.8239 = 13.2 |
||||||||||||
| 16 | B |
The titration curve depicts equivalence factors at roughly 10.0 mL and 20.0 mL. Since there are two equivalence factors, the acid is probably going diprotic. |
||||||||||||
| 17 | D | Primarily based on the mole ratio of phosphate in every compound:
n(Na3PO4) = n((NH4)3PO4•12MoO3) n((NH4)3PO4•12MoO3) = m / MM = 24.21 / 1877 = 0.012898 mol = n(Na3PO4) m(Na3PO4) = n x MM = 0.012898 x (3 x 22.99 + 30.97 + 4 x 16.00) = 2.115 g |
||||||||||||
| 18 | D |
|
||||||||||||
| 19 | C | 2AgNO3(aq) + Ok2SO4(aq) Ag2SO4(s) + 2KNO3(aq)
Ag2SO4(s) ⇋ 2Ag+(aq) + SO42-(aq) Oksp = [Ag+]2[SO42-] = 1.20 x 10-5 → [Ag+] = 1.20 10-50.100 = 0.01095 mol L-1 n(Ag+) = c x V = 0.01095 x 0.250 = 0.002739 mol = n(AgNO3) m(AgNO3) = n x MM = 0.002739 x 169.9 = 0.465 g |
||||||||||||
| 20 | C | [(CH3)3NH]+(aq) + H2O(l) ⇋ H3O+(aq) + (CH3)3N(aq)
Oka = [H3O+][(CH3)3N] / [[(CH3)3NH]+] = 1.55 x 10-10 pH = 4.46 → [H3O+] = 10-4.46 = [(CH3)3N] (based mostly on stoichiometry) [[(CH3)3NH]+] = [H3O+][(CH3)3N] / Oka = [10-4.46][10-4.46] / 1.55 x 10-10 = 7.7565 mol L-1 In a saturated answer of trimethylammonium chloride: [(CH3)3NH]Cl(s) ⇋ [(CH3)3NH]+(aq) + Cl–(aq) Oksp = [[(CH3)3NH]+][Cl–] [Cl–] = [[(CH3)3NH]+] = 7.7565 mol L-1 (based mostly on stoichiometry) Due to this fact, Oksp = (7.7565)(7.7565) = 60.2 |
Part 2: Lengthy Response Questions
Query 21(a)
Security concern: Natural liquids are unstable and flammable, which means they’ll catch on hearth and maintain combustion. To deal with: guarantee there aren’t any open flames and steer clear of ignitable sources. Work in fume hood.
Query 21(b)
Flask 1: Propanoic acid, flask 2: hex-1-ene, flask 3: propan-1-ol, flask 4: hexane
Query 21(c)
Hex-1-ene: bromine check. Speedy decolourisation of bromine from red-brown to colourless could be noticed.
Query 22
Warmth the answer/Add NaOH.
Addition of warmth disturbs the equilibrium. Based on Le Chatelier’s precept, the equilibrium shifts left within the endothermic course to take away added warmth and minimise the disturbance.
Addition of NaOH, leads to a lower in focus of H+ as OH–(aq) + H+(aq) H2O(l). This disturbs the equilibrium. Based on Le Chatelier’s precept, the equilibrium shifts left in to switch H+ and minimise the disturbance.
Query 23(a)
HCOOH(aq) + KOH(aq) KHCOO(aq) + H2O(l)
Query 23(b)
Fundamental.
Dissolving: KHCOO(s) Ok+(aq) + HCOO–(aq)
Reacting with water: HCOO–(aq)+ H2O(l) ⇌ HCOOH(aq) + OH–(aq)
KHCOO dissolves and produces HCOO–(aq) which is primary as it’s the conjugate base of a weak acid. It reacts with water to provide OH–(aq), rising the pH and forming a primary answer.
Query 24
|
Structural formulation |
Identify |
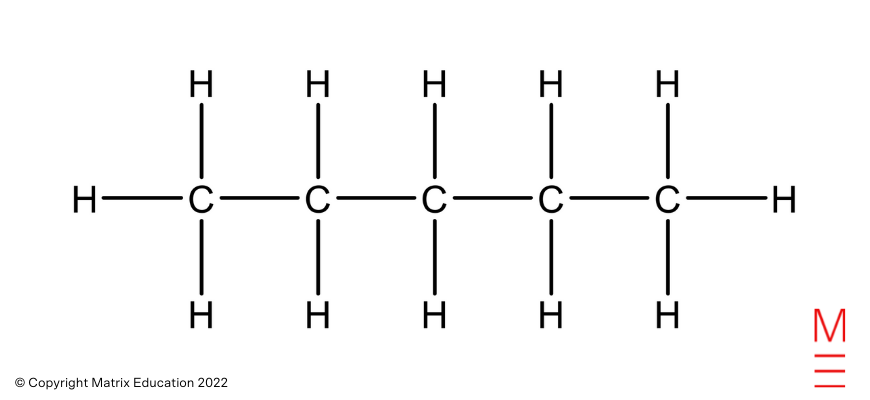 |
Pentane |
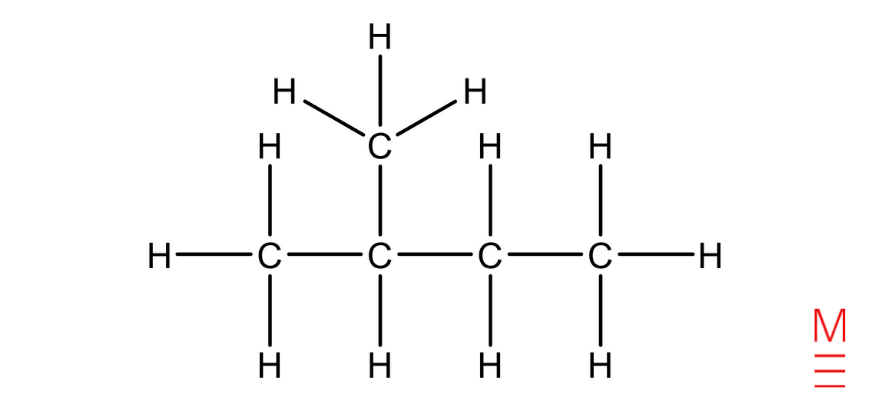 |
2-methylbutane |
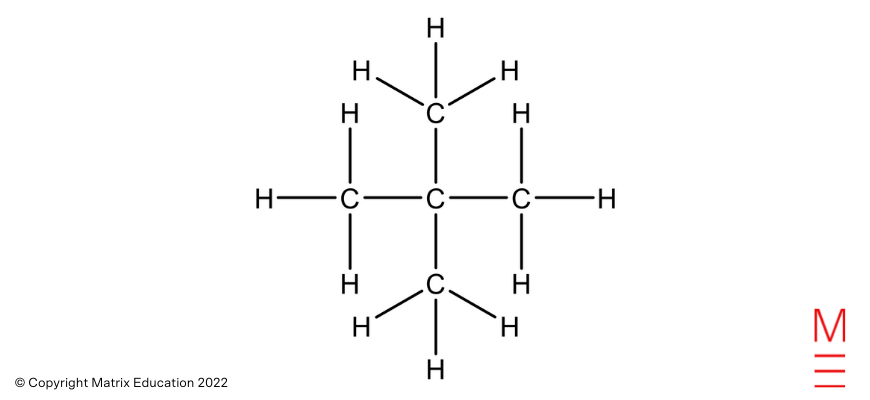 |
2,2-dimethylpropane |
Query 25
C6H12O6(aq) 2C2H5OH(aq) + 2CO2(g)
V(CO2) = 1006 mL = 1.006 L
n(CO2) = V/MV = 1.006 / 24.79 = 0.040580 mol
n(C2H5OH) = n(CO2) = 0.040580 mol
m(C2H5OH) = n x MM = 0.020855 mol x (12.01 x 2 + 1.008 x 6 + 16.00) g mol-1 = 1.87 g (3 s.f)
Query 26(a)
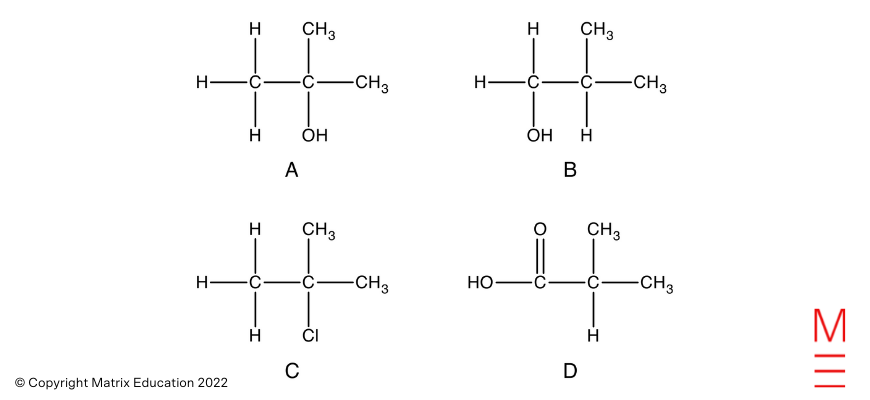
Query 26(b)
Esterification is gradual at room temperature, therefore heating is required to extend the speed. The reactants and merchandise are additionally unstable. Therefore reflux is used to permit for light heating with out the lack of reactants and merchandise.
Query 27(a)
Oksp = [Li+]3[PO43-]
Precipitation happens between pattern 3 and 4.
Pattern 3:
Decrease Oksp = 0.153 x 0.01 = 3.4 x 10-5 (2 s.f.)
Pattern 4:
Increased Oksp = 0.153 x 0.10 = 3.4 x 10-4 (2 s.f.)
Vary = 3.4 x 10-5 to three.4 x 10-4
Query 27(b)
Extra increments within the focus of phosphate could possibly be used which might permit the vary to be narrowed.
Alternatively, flip into precipitation titration.
Query 28
2MOH(aq) + Cu(NO3)2(aq) Cu(OH)2(s) + 2MNO3(aq)
n(Cu(OH)2) = m / MM = 4.61 g / (63.55 + 16.00 x 2 + 1.008 x 2) g mol-1 = 0.04725 mol
n(MOH) = 2 x n(Cu(OH)2) = 0.0945 mol
MM(MOH) = m(MOH) / n(MOH) = 5.30 g / 0.0945 mol = 56.084577 g mol-1
MM(M) = MM(MOH) – MM(OH) = 56.084577 – (16.00 + 1.008) = 39.1 g mol-1 (3 s.f.)
Alkali metallic hydroxide = Potassium hydroxide, KOH
Query 29
IR Spectrum: The sturdy absorption between 3000 – 3700 cm-1 is attribute of N-H bonds in amines. That is current in pentane-1,5-diamine.
Mass Spectrum: The fragment ion with a m/z ratio of 30 corresponds to
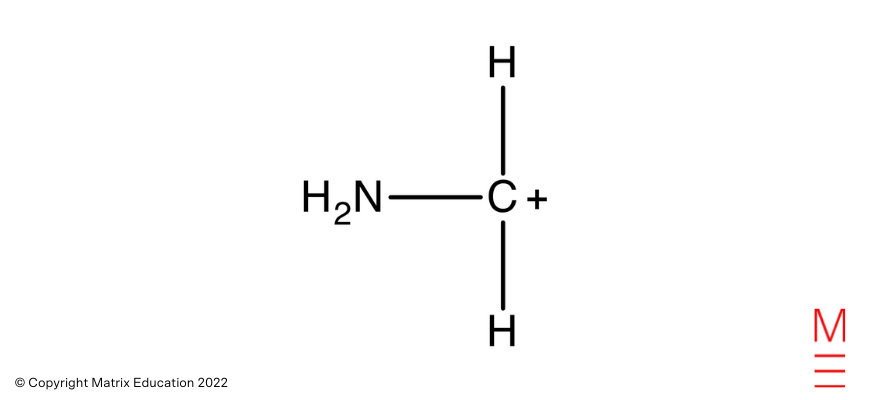
Which is obtained from the fragmentation of pentane-1,5-diamine as proven
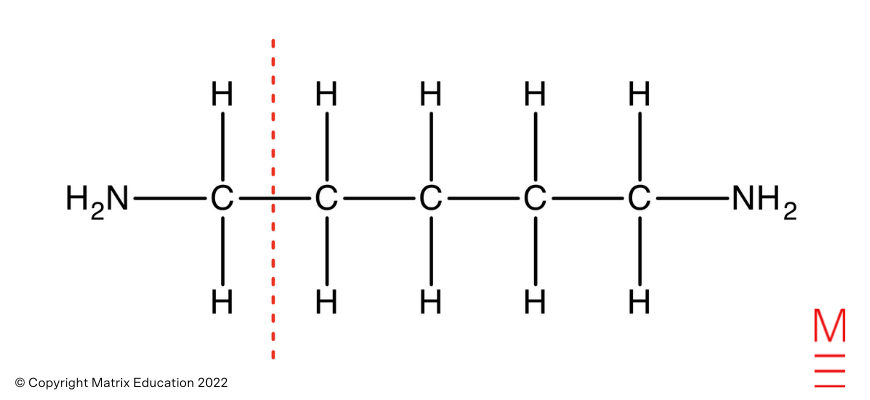
Carbon-13 NMR Spectrum: There are three peaks within the 13C NMR spectrum, representing 3 distinctive carbon environments. That is in step with pentane-1,5-diamine as proven:
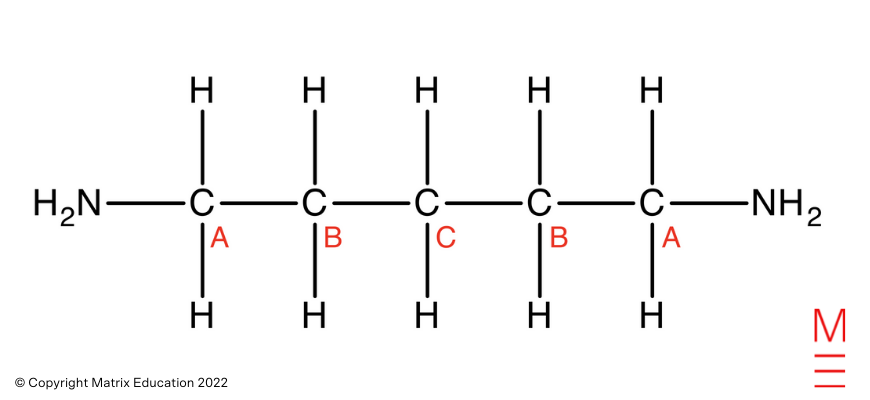
“A” carbons correspond to probably the most downfield peak, which falls inside the attribute chemical shift vary for carbons straight bonded to amines. The “A” carbons are straight bonded to an electronegative nitrogen which withdraws electron density from the carbons. This causes these carbons to be deshielded and expertise extra of the exterior magnetic discipline, therefore absorbs the next frequency of radiation.
“B” carbon corresponds to the center peak, which falls inside the attribute chemical shift vary for aliphatic carbons. Carbon B is the one carbon away from the amine, therefore will probably be extra shielded than the A carbons. Due to this fact, the B carbons expertise much less of the exterior magnetic discipline and take in a decrease frequency of radiation.
“C” carbon corresponds to probably the most upfield peak, which falls inside the attribute chemical shift vary for aliphatic carbons. Carbon C is the furthest away from the amine, therefore will probably be most shielded and subsequently will expertise much less of the exterior magnetic discipline. Consequently, Carbon C absorbs a decrease frequency of radiation.
Proton NMR Spectrum: The overlapping quintets correspond to the hydrogens on B carbons and C carbon. The quintet splitting signifies that the hydrogens neighbour 4 chemically equal protons in a special chemical surroundings.
Query 30
The addition of aqueous sodium chloride is just not helpful for figuring out any of the ions. Na+ ion doesn’t precipitate with OH– or CH3COO– ions. Equally, Cl– ion doesn’t precipitate out Ba2+, Ca2+ or Mg2+ ions.
The addition of aqueous silver nitrate is beneficial for distinguishing between OH– and CH3COO– ions. Silver ions react with OH– ions to provide a brown precipitate, which dissolves in dilute HCl. Though acetate ions can precipitate with silver ions, the precipitate is much less soluble and is white, thus might be simply distinguished.
The addition of aqueous sodium sulfate is just not a helpful check for use alone. The sulfate ion can precipitate out each Ba2+ and Ca2+ ions, and each precipitates are white and can’t be distinguished visually. An extra check, like a flame check, is required to differentiate between Ba2+ and Ca2+ ions. The check permits for Mg2+ ions to be eradicated, however can not definitively determine its presence.
Query 31
|
Focus (M) |
N2(g) |
3H2(g) |
2NH3(g) |
|
Preliminary |
0.45 + x |
0.1 |
0.58 |
|
Change |
– 0.0025 |
– 0.0075 |
+ 0.005 |
|
Equilibrium |
0.4475 + x |
0.0925 |
0.585 |
()
start{align*}
Ok = frac{[NH_3]^2 }{[N_2][H_2]^3 } &= 748
frac{0.5852}{((0.4475 + x) occasions 0.09253} &= 748
frac{0.5852}{3.5417} occasions 10-4 + 0.09253x &= 748
0.5852 &= 748 (3.5417 occasions 10-4 + 0.09253x)
0.5852 &= 0.264923 + 0.592006x
x = 0.5852 – frac{0.264923}{0.592006} &= 0.130576 textual content{mol /L}
finish{align*}
To 10 L, add 1.3 mol (2 s.f.)
Query 32
In each reactions 1 and a couple of, a powerful acid and powerful base are reacted collectively. A powerful acid utterly ionises in water to provide H+ ions and a powerful base utterly dissociates to provide OH– ions. Thus the enthalpy change solely displays the response between H+ and OH– to provide water. OH–(aq) + H+(aq) H2O(l)
For the reason that web response is similar, ΔH is similar.
In response 3, a weak acid is reacted with a powerful base. A weak acid is just not absolutely ionised in water, therefore some intact acid will probably be current. Throughout neutralisation, power from the response will probably be absorbed to interrupt the covalent bond within the intact acid to type H+ ions. The enthalpy change subsequently displays each the exothermic response between H+ and OH– and the endothermic bond breaking course of. Therefore general ΔH will probably be much less exothermic.
Query 33(a)
ΔG = ΔH – TΔS = -95 – (-80) = -15 kJ/mol
Query 33(b)
At T1, the ΔH < TΔS. Since ΔG = ΔH – TΔS, ΔG < 0. Therefore the response is spontaneous within the ahead course and non-spontaneous within the reverse course at this temperature.
At T3, the ΔH > TΔS. Since ΔG = ΔH – TΔS, ΔG > 0. Therefore the response is non-spontaneous within the ahead course and spontaneous within the reverse course at this temperature.
At T2, the ΔH = TΔS. Since ΔG = ΔH – TΔS, ΔG = 0. Therefore the system is at equilibrium at this temperature.
Query 34
The pH of water is initially at 7 as it’s impartial. As gaseous HCl is bubbled into the water, it dissolves and ionises to provide H3O+ ions.
HCl(aq) + H2O(l) H3O+(aq) + Cl–(aq)
As extra HCl bubbles into the water, the focus of H3O+ will increase. Since pH = -log10[H3O+], the pH of the answer decreases with rising focus of H3O+. The change within the pH between t0 and t1 is steep because the proportional change within the focus of H3O+ ions is initially giant. The change in pH between t1 and t2 is much less steep because the proportional change within the focus of H3O+ ions is much less as extra acid is added.
The pH of X and Y might be noticed to steadily lower between t0 and t1. This means X and Y are buffer options, containing a weak acid/base and its conjugate. For the reason that pH of those options at t0 is round pH 5, they’re probably to be composed of a weak acid and the conjugate base.
HA(aq) + H2O(l) ⇌ H3O+(aq) + A–(aq)
When HCl is added and the focus of H3O+ will increase, these options are in a position to withstand modifications within the focus of H3O+ because the equilibrium will shift left to take away the added H3O+ to minimise the disturbance, in accordance with Le Chatelier’s precept. Since focus of H3O+ is comparatively unchanged, pH can be comparatively unchanged.
In between t1 and t2, the pH decreases extra quickly for each X and Y, indicating the options have reached their buffering capability. Resolution X is depleted prior to Y suggesting X has a decrease buffering capability in comparison with Y. This can be because of X having a decrease focus of weak acid and its conjugate base, in comparison with Y.
Query 35
V(Na2S2O3) = (28.7 + 28.4 + 28.6) / 3 = 28.56667 mL = 0.02856667 L
n(Na2S2O3) = c x V = 0.900 x 0.02856667 = 0.02571 mol = n(S2O32-)
n(I2) = ½ x n(S2O32-) = 0.012855 mol
n(Cr2O72-)extra = ⅓ x n(I2) = 0.004285 mol
n(Cr2O72-)whole = c x V = 0.500 x 0.020 = 0.010 mol
n(Cr2O72-)reacted = 0.010 – 0.004285 = 0.005715 mol
n(C2H5OH) = 3/2 x n(Cr2O72-)reacted = 8.5725 x 10-3 mol
c(C2H5OH)diluted = 8.5725 x 10-3 / 0.025 = 0.3429 mol L-1
c(C2H5OH)concentrated = (0.3429 x 1.00) / 0.025 = 13.716 mol L-1
In 1 L:
n(C2H5OH) = 13.716 mol
m(C2H5OH) = n x MM = 13.716 x (12.01 x 2 + 1.008 x 6 + 16.00) = 631.868 g
V(C2H5OH) = 631.868 / 0.789 = 800.8475 mL
% v/v = (800.8475 / 1000) x 100 = 80.1 % v/v (3 s.f.)
Lower than 85%, thus doesn’t meet producer’s necessities.
Query 36
Response 1: H2SO3(aq)+ H2O(l) ⇌ H3O+(aq) + HSO3–(aq)
Oka1 = [H3O+][HSO3–] / [H2SO3] = 10-1.82
Response 2: HSO3–(aq)+ H2O(l) ⇌ H3O+(aq) + SO32-(aq)
Oka2 = [H3O+][SO32-] / [HSO3–] = 10-7.17
Response 3: H2SO3(aq)+ 2H2O(l) ⇌ 2H3O+(aq) + SO32-(aq)
Oka3 = [H3O+]2[SO32-] / [H2SO3]
Oka1 x Oka2 = [H3O+][HSO3–] / [H2SO3] x [H3O+][SO32-] / [HSO3–] = [H3O+]2[SO32-] / [H2SO3]
Due to this fact,
Oka3 = Oka1 x Oka2 = 10-1.82 x 10-7.17 = 1.0 x 10-9 (2 s.f.)
,