The 2018 HSC Chemistry exam is done and dusted. While the official exam paper has not yet been released by NESA, here are our draft suggested solutions and some explanations in case you wanted to know ASAP! Read on for the 2018 HSC Chemistry Exam Paper solutions.
HSC Chemistry Section I A (Multiple Choice) Solutions
| Question | Solution | Explanation |
| 1 | B | Ammonia’s main use (almost 90%) is in fertilisers. |
| 2 | A | Chlorine is used in water treatment to kill microorganisms (disinfection) |
| 3 | C | Esterification is a reversible reaction that reaches equilibrium. Adding more alkanol (a reactant) would shift the equilibrium to the right (Le Chatelier’s principle) |
| 4 | B | Trace elements are found at low concentrations that can be detected using AAS. |
| 5 | D | Cellulose contains a basic carbon-chain structure that can be utilised to form polymers e.g. via hydrolysis and fermentation. |
| 6 | B | Sodium hydrogen carbonate is amphiprotic (donates and accepts protons) so it can be used to neutralise both acids and alkalis. It is also stable (useful for storage) and easily handled (as it is a solid). |
| 7 | C | A weak acid partially ionises in water, therefore the beaker should contain hydronium, anions and water. |
| 8 | B | Mineral smelting produces SO2. A is incorrect as volcanoes produce SO2, C is incorrect as it shows the formation of an acid, not the acidic oxide. D is incorrect as CO is not acidic. |
| 9 | D | Lavoisier defined acids as oxygen-containing species. HCl does not contain oxygen. |
| 10 | D | Dehydration occurs when an alkanol reactant is converted into an alkene and water using a concentrated acid catalyst. |
| 11 | A | PVC’s chlorine side group makes it rigid. |
| 12 | A | Silver is oxidised from 0 (as elemental silver, Ag) to +1 (in Ag2S) |
| 13 | C | The compound with the highest boiling point would remain in the flask. This is pentanoic acid as it has the strongest intermolecular forces (stronger hydrogen bonding than the alkanol). Note: propyl acetate is another name for the ester propyl ethanoate. |
| 14 | C | The possible isomers are:
|
| 15 | D | Dihydrogen phosphate is H2PO4–, hydrogen phosphate is HPO42-. When acid is added, equilibrium will shift left to remove H3O+. The opposite occurs when alkali is added. |
| 16 | A | n(CaCO3) = m / MM = 0.4 / 100.09 = 0.0039964 mol = n(Ca2+) in 2 L
n(Ca2+) in 1 L = 0.0019982 mol m(Ca2+) in 1 L = 0.080 g Therefore concentration is 80 mg L-1 |
| 17 | A | A buffer resists changes in pH, so the solution with the buffer would have a gradual decrease in pH, followed by a steeper decrease. The pH of the water beaker would decrease faster (lower pH at all concentrations). |
| 18 | C | [H+] = 10-2.9 = 0.0012589 M
% = [H+]/[HA] x 100 = 0.0012589 / 0.08 x 100 = 1.6% |
| 19 | D | Highest reading means largest potential difference. A and B are set up so the reactants would be the same metal element. C would give a reading of 0.43 V, D would give a reading of 0.78 V. |
| 20 | B | Using the mole ratio in the Winkler titration equations given, work backwards:
n(O2) in 5 L = 1 x 10-3 mol, therefore 0.2 x 10-3 mol in 1 L m(O2) in 1 L = n x MM = 0.2 x 10-3 x 32 = 0.0064 g = 6.40 mg L-1 |
HSC Chemistry Section I B (Long Response) Solutions
| Question | Marks | Solution |
| 21(a) | 1 | Cracking |
| 21(b) | 2 | 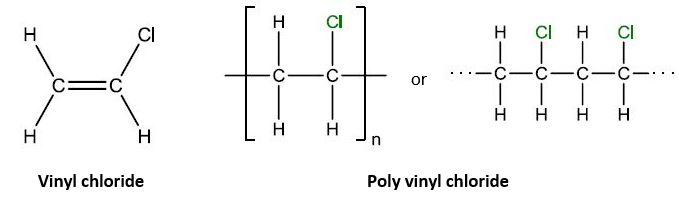 |
| 22 | 4 |
If the unknown solution is Pb(NO3)2, all three test tubes would have a white precipitate.
If the unknown solution is Ba(NO3)2, test tubes B and C would have a white precipitate, A would have no reaction.
If the unknown solution is Fe(NO3)2, test tube B would have a greenish precipitate, A and C would have no reaction.
|
| 23(a) | 2 | 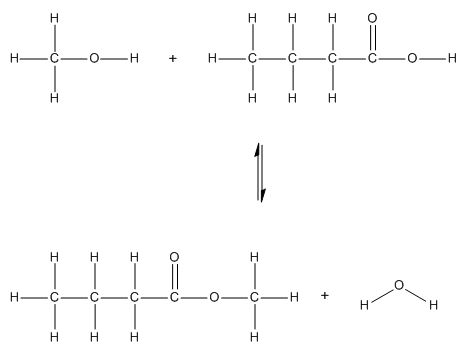 |
| 23(b) | 4 |
|
| 24(a) | 1 | C6H12O6(aq) → 2C2H5OH(aq) + 2CO2(g) |
| 24(b) | 3 | V(CO2) = 5.5 / 1000
n(CO2) = V / MV = 0.0022186 mol n(C6H12O6) = 0.00011093 mol m(C6H12O6) = 0.00011093 x 180.156 = 0.019985 g = 0.020 g (2 s.f.) |
| 25 | 4 | The rate of reaction depends on the frequency of successful collisions (exceeding activation energy).
Changing temperature When temperature is increased, the kinetic energy of the particles increases. This means a greater proportion of the N2 and H2 present will exceed activation energy and react, increasing the rate of reaction. This can be seen as the area under the curve that is to the right of EA i.e. the number of molecules that can react is larger at T2 than T1. Adding a catalyst A catalyst provides an alternate reaction pathway with a lower activation energy. This means a greater proportion of collisions will have sufficient energy to react. On the graph above, the EA for a catalysed reaction would be further to the left, therefore a greater area under the curve would be to the right of the catalysed EA at both temperatures. |
| 26(a) | 2 | A Geiger counter has a gas-filled metal tube containing a wire at a high positive potential. Ionising radiation entering the tube through a thin window ionises some of the gas. The electrons produced are attracted to the wire. This produces a current which is converted to a clicking sound. |
| 26(b) | 5 | Technetium-99m
Benefits to society:
Problems to society associated with its use
|
| 27(a) | 1 | 2-chloro-1,1-difluoroethane
The substituents are ordered alphabetically. The numbers are determined by the first point of difference rule in line with the IUPAC system (not the lowest sum, although this coincidentally gives the same answer). |
| 27(b) | 4 | CFCs are useful in aerosols due to their low toxicity and low reactivity. However, when they diffuse into the stratosphere, they destroy ozone catalytically which causes an increase in cancer and cataract-causing UV at ground level.
In the stratosphere, high energy UV causes the photodissociation of CFCs, producing a Cl• radical e.g. for CFC-12: CCl2F2(g) → CClF2(g) + Cl•(g) The Cl• radical then breaks down ozone: Cl•(g) + O3(g) → ClO•(g) + O2(g) The Cl• radical is regenerated so it can continue to destroy more ozone: ClO•(g) + O•(g) → Cl•(g) + O2(g) The compound above is a HCFC. HCFCs contain a reactive C-H bond, so most HCFC molecules are decomposed by free radicals in the lower atmosphere before reaching the upper atmosphere. However a small proportion will still reach the stratosphere to destroy ozone (since they still contain Cl) and hence they still will destroy ozone. A more environmentally friendly replacement for CFCs are the HFCs. Like HCFCs they contain a C-H bond so they readily decompose in the troposphere, but they contain no Cl so they cannot generate Cl radicals and destroy ozone. However, they are more expensive than CFCs and are less efficient in its industrial applications. |
| 28(a) | 3 | 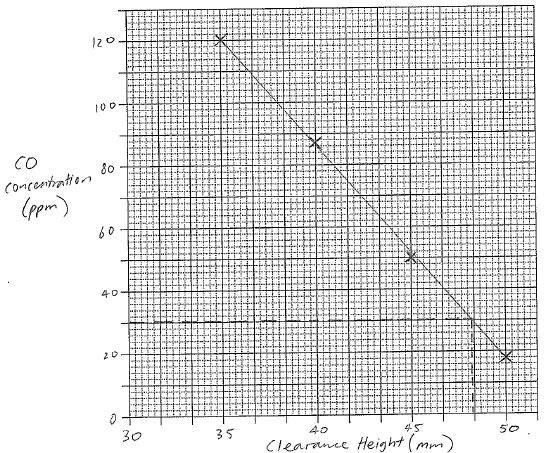 |
| 28(b) | 1 | 48 mm |
| 28(c) | 4 | Lowest safe distance = 48 mm
Each mm increase = 4% efficiency decrease, therefore maximum efficiency = 38% n(C4H10) = m / MM = 15 / 58.12 = 0.2580867 mol q = n x ∆H = 0.2580867 x 2877 = 742.5155 kJ 38% of this is 742.5155 x 0.38 = 282.1559 kJ q = mC∆T ∆T = q / mC = (282.1559 x 103) / (1 x 4.18 x 103) = 67.5 K Tfinal = 20 + 67.5 = 87.5 = 88 °C (2 s.f.) |
| 29(a) | 2 | Sodium carbonate is stable in air, solid, easily obtainable in a pure form, highly soluble in water and has a high molecular mass, therefore it can be used to prepare a solution of a known concentration accurately. |
| 29(b) | 3 | Average volume is 21.65 mL (22.00 mL is an outlier)
Na2CO3(aq) + 2HCl(aq) → 2NaCl(aq) + CO2(g) + H2O(l) n(Na2CO3) = c x V = 0.105 x 0.025 = 0.002625 mol n(HCl) = 0.002625 x 2 = 0.00525 mol c(HCl) = n / V = 0.242494 M = 0.2425 M (4 s.f.) |
| 29(c) | 2 | Phenolphthalein changes colour at a higher pH (8.2 – 10.0) than methyl orange (3.1 – 4.4). Therefore less acid would be added to the flask if phenolphthalein was used, resulting in a higher calculated concentration of HCl. |
| 30 | 7 | CO2(g) ⇌ CO2(aq) ∆H < 0
Factors that affect this equilibrium:
When more fossil fuels are burnt, more carbon dioxide gas is produced. For example, the combustion of octane (C8H18) is shown below: C8H18(l) + 12.5O2(g) → 8CO2(g) + 9H2O(l) Hence the atmospheric carbon dioxide levels would increase. Atmospheric carbon dioxide is a greenhouse gas which causes the Earth to warm up, hence air and ocean temperatures have increased. Increased temperature would also cause the equilibrium above to shift to the left, causing the proportion of CO2 in the atmosphere to increase relative to dissolved CO2 . This contributes to the increase in atmospheric CO2 levels. However, since there is still greater carbon dioxide overall due to the increased burning of fossil fuels, the volume of CO2 dissolved in the oceams has still increased as well. |
HSC Chemistry Section II (Option Topic) Solutions
Question 31: Industrial Chemistry
| Question | Marks | Solution | ||||||||||||||||||||
| 31(a)(i) | 2 | 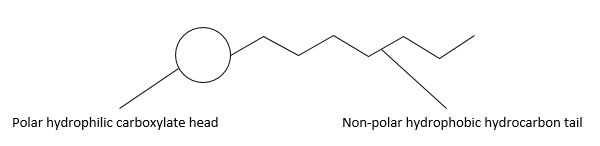 |
||||||||||||||||||||
| 31(a)(ii) | 3 | Soaps function by both reducing the surface tension of water and by binding to grease and/or dirt. They consist of a charged anionic head group and non-polar hydrocarbon chain.
The hydrophilic head of a soap ion interacts with water molecules via ion-dipole interactions and hydrogen bonding.Grease and oil consist of non-polar molecules. The hydrophobic tails of the soap molecules dissolve in the grease. With agitation, the grease layer breaks up into smaller, spherical droplets, with the hydrophilic surfactant head groups sticking out into the water and the hydrophobic surfactant tails adsorbed into the grease, leaving a clean surface. |
||||||||||||||||||||
| 31(b)(i) | 2 | Concentrated sulfuric acid reacts (ionises) with water exothermically. Therefore it must be transported and stored in waterproof containers. | ||||||||||||||||||||
| 31(b)(ii) | 4 | Oxidising agent:
Dehydrating agent:
|
||||||||||||||||||||
| 31(c)(i) | 3 | Add sodium chloride: When the concentration of Cl–(aq) increases, the equilibrium is disturbed. According to Le Chatelier’s principle, equilibrium shifts right to remove the added Cl–(aq), minimising the disturbance. Therefore more of the blue solution will be formed.
Heat up the solution: When temperature increases, the equilibrium is disturbed. According to Le Chatelier’s principle, equilibrium shifts to the right in the endothermic direction to use up heat and minimise the disturbance. Therefore more of the blue solution will be formed. |
||||||||||||||||||||
| 31(c)(ii) | 4 |
K = [HI]2 / ([H2][I2]) 64 = (2x)2 / (0.2-x)2 Square root both sides: 8 = 2x / (0.2 – x) 8(0.2 – x) = 2x 1.6 – 8x = 2x 10 x = 1.6 x = 0.16 Therefore the equilibrium concentration of HI is 2x = 0.32 M |
||||||||||||||||||||
| 31(d) | 7 | Sodium hydroxide
Several methods can be used for the production of sodium hydroxide: the mercury, diaphragm and membrane processes. These processes have the following overall electrolysis reaction: 2NaCl(aq) + 2H2O(l) → H2(g) + Cl2(g) + 2NaOH(aq) Each process has its own specific environmental concerns. The diaphragm cell is associated with the leakage of asbestos waste, which if inhaled can potentially cause lethal lung diseases and harm surrounding wildlife. In the mercury cell, although the mercury is recycled, there is an inevitable loss of mercury to the environment. Mercury is a toxic heavy metal that can cause neurological damage to wildlife and humans, especially higher order predators like large fish and mammals as it biomagnifies in the food chain. Monitoring can help address these concerns, but the main method has been the replacement of the mercury and diaphragm processes by the membrane cell, which uses a synthetic polymer membrane instead of asbestos. It also does not require the use of mercury. This effectively addresses the environmental issues associated with the older processes. The high energy requirement for sodium hydroxide production also means there is significant consumption of fossil fuels, which is a contemporary environmental and social issue with regard to global warming and the oil crisis. This has also been partially addressed by the membrane cell, which requires less energy to run than the older processes. Switching to renewable energy can help as well. Sodium carbonate Sodium carbonate can be produced industrially via the Solvay process: 2NaCl(aq) + CaCO3(s) → Na2CO3(s) + CaCl2(aq) The major environmental concerns are:
|
Question 32: Shipwrecks, Corrosion and Conservation
| Question | Marks | Solution | ||||||||||||
| 32(a)(i) | 2 | Amount of current, surface area of electrodes, distance between electrodes | ||||||||||||
| 32(a)(ii) | 3 |
|
||||||||||||
| 32(b)(i) | 2 |
|
||||||||||||
| 32(b)(ii) | 4 | The corrosion of iron proceeds as follows:
1. Iron oxidises at the anode. The electrons flow through the iron to the cathode, which is an impurity in the iron such as carbon. Fe(s) → Fe2+(aq) + 2e- 2. Reduction of oxygen gas at the cathode. The electrons provided from the oxidation of iron reduces oxygen that is dissolved in the moisture on the surface of iron to form hydroxide ions. O2(g) + 2H2O(l) + 4e– → 4OH–(aq) 3. Precipitation of iron(II) hydroxide. Fe(II) ions readily combine with hydroxide ions to form iron(II) hydroxide. Fe2+(aq) + 2OH–(aq) → Fe(OH)2(s) 4. Formation of rust. Iron(II) hydroxide is easily oxidised to iron(III) by oxygen to form rust. 4Fe(OH)2(s) + O2(g) → 2Fe2O3.H2O(s) + 2H2O(l) The overall reaction is: 4Fe(s) + 3O2(g) + 2H2O(l) → 2Fe2O3·H2O(s) |
||||||||||||
| 32(c)(i) | 3 | The presence of sulfate-reducing bacteria (SRB) is predominately responsible for the formation of rusticles found on the Titanic. These anaerobic bacteria thrive by reducing sulfate ions that are highly abundant in the ocean. The electrons required for these reductions are supplied by oxidation of metals such as iron and silver. This occurs as follows:
Reduction: SO42-(aq) + 5H2O(l) + 8e– → HS–(aq) + 9OH–(aq) Oxidation: Fe(s) → Fe2+(aq) + 2e– and Ag(s) → Ag+(aq) + e– The hydrogen sulfide ion is the SRB’s waste product. Once expelled, it can dissociate to sulfide ions and hydrogen ions: HS–(aq) ⇌ H+(aq) + S2-(aq) The oxidised metal can react with the sulfide ions and hydroxide ions to form the corrosion products iron(II) sulfide, iron(II) hydroxide and silver sulfide. Fe2+(aq) + S2-(aq) → FeS(s) Fe2+(aq) + 2OH–(aq) → Fe(OH)(s) 2Ag+(aq) + S2-(aq) → Ag2S(s) |
||||||||||||
| 32(c)(ii) | 4 | Encrusted silver coins salvaged from wrecks can be restored to the original structure by electrochemical reduction of the metal ions to the metals, either through galvanic reduction or electrolytic reduction.
In galvanic reduction, the silver ions in the corrosion layers are reduced back to the silver metal by making the silver coin the cathode by connecting it to a more active anodic metal such as aluminium. Both metals in contact form a galvanic cell and the following reactions occur: Ag2S(s) + 2e– → 2Ag(s) + S2-(aq) Al(s) → Al3+(aq) + 3e– While galvanic reduction is a spontaneous redox reaction that can be sped up by applying heat, electrolytic reduction requires use of a direct electric current (DC) to drive an otherwise non-spontaneous redox chemical reaction. In electrolytic reduction, the silver coin is made the cathode and is connected to an inert anode such as stainless steel. The process at the cathode is: Ag2S(s) + 2e– → 2Ag(s) + S2-(aq) Hydroxide ions are oxidised at the anode: 4OH–(aq) → O2(g) + 2H2O(l) + 4e– |
||||||||||||
| 32(d) | 7 | Advances in chemistry have allowed continued improvements to methods used in the protection of steel ships from corrosion in marine environments. Current methods of protection include surface coatings, use of sacrificial anode systems or impressed current systems. Knowledge that corrosion occurs from the contact of the metal with oxygen and water has led to the use of paints as a form of protection. The paint is applied to the entire ship hull and acts as a physical barrier between the steel hull and oxygen and water. However, the paints needed to be reapplied periodically as abrasion and exposure of the underlying metal led to corrosion. Through the understanding of importance of ion flow in the process of corrosion, specialized polymer paints that actively prevent the migration of ions were developed to prevent corrosion even when the metal surface is exposed to oxygen and water. These polymer paints cure in air to form a film that is impervious to oxygen and water, and contain additives that form a tight ionic layer on the steel surface which prevents movement of ions, thus prevents corrosion.
Further understanding of redox chemistry and metal reactivity led to the development of cathodic protection techniques, whereby the steel ship hull is protected by connecting it as the cathode, so that the metal will have a constant supply of electrons. This ensures that any Fe2+ ion formed would be reduced back to the metallic atom, thereby preventing its corrosion. Fe2+(aq) + 2e– → Fe(s) There are two methods of cathodic protection. One is the use of the sacrificial anode system, which sets up a galvanic cell by attaching the steel hull to a more reactive metal such as zinc. The more reactive metal has a greater oxidation potential than iron. Zn(s) → Zn2+(aq) + 2e– Eox: 0.76 V Fe(s) → Fe2+(aq) + 2e– Eox: 0.44 V Thus it will oxidise in preference to iron and act as the anode, thereby forcing the iron to be the cathode. This process is effective as long as the anode is periodically replaced before it completely corrodes away. The second method is the use of impressed current systems. An electrolytic cell is set up by connecting the metal ship hull to the negative terminal of an external power source as the cathode and an inert metal such as platinum is connected at the positive terminal as the anode. A low voltage is continuously supplied. This ensures the steel ship hull is provided electrons continuously to reduce any Fe2+ ions back to metallic iron. |
Question 34: The Chemistry of Art
| Question | Marks | Solution |
| 34(a)(i) | 2 | 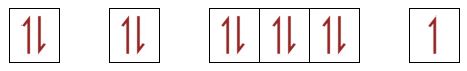 |
| 34(a)(ii) | 3 | The energies of the subshells in the 3rd and 4th shells overlap. The energy of the 4s subshell is lower than the energy of the 3d subshell. Therefore due to the Aufbau principle (subshells with lower energies fill first), electrons fill the 4s subshell before the 3d subshell. This can be seen in the first row of transition metals: the 4s subshell is filled before the 3d subshell is complete. However, after the 3d subshell is complete, then other subshells in the 4th shell will fill (4p, 4d, 4f) as they have higher energy. |
| 34(b)(i) | 2 | 5 mL of potassium permanganate was added dropwise to 20 mL of potassium iodide in a test tube. The colour changes were recorded. The procedure was repeated using potassium bromide, potassium chloride and potassium fluoride in place of potassium iodide. |
| 34(b)(ii) | 4 | Observed results: The deep purple colour of MnO4–(aq) turned into pale pink Mn2+ for KI, KBr and KCl. However, no colour change occurred for KF.
One conclusion: Potassium permanganate is a strong enough oxidant to oxidise iodide ions. MnO4–(aq) + 8H+(aq) + 5e– → Mn2+(aq) + 4H2O(l) 2I–(aq) → I2(s) + 2e– |
| 34(c)(i) | 3 | An absorption spectrum is produced when electrons absorb photons of certain wavelengths and become excited from lower to higher quantised energy levels or orbits. The absorption spectrum appears as black lines against a bright background.
An emission spectrum is produced when electrons that have been excited to higher orbits emit photons as they transition to lower orbits. The emission spectrum is seen as bright coloured lines on a dark background. |
| 34(c)(ii) | 4 | Pigments absorb wavelengths of infrared and ultraviolet which match differences between quantised energy levels. The absorbed frequencies can be identified via absorption or reflectance spectroscopy (wavelengths not absorbed are reflected). The collected frequencies can be plotted as wavelength vs intensity to produce a spectrum, and compared with a database of reference spectra to determine the pigments present.
IR corresponds to vibrations of covalent bonds, and is mostly useful for identification of pigments containing covalent bonds (organic pigments and those containing polyatomic ions like indigo and Prussian blue, particularly copper pigments) and carbon-based pigments like graphite. Additionally, infrared can penetrate into the underlayers so it can be used to non-destructively analyse pigments used in the underdrawings. UV corresponds to electronic transitions, and is useful for many pigments. Additionally, UV fluorescence can occur with some pigments which release the absorbed UV energy in the visible region, and the visible wavelengths can also be used to produce a spectrum. To determine the concentration of pigments, the absorbance or reflectance of the pigment can be used. The greater the absorbance or reflectance, the higher the concentration of the pigment. |
| 34(d) | 7 | Aboriginal people
Pigments obtained from sources like rocks or charcoal were ground to a fine powder with a stone tool, then mixed with a suitable medium such as water, wax or resin. It was then painted onto cave walls or skin with a brush. Pigments could also be blown onto wet surfaces to create stencil art. Examples of pigments used by Australian Aboriginal people:
Ancient Egyptians Similar processes were used by ancient Egyptians to prepare and attach pigments to surfaces. However, there was more widespread use of pigments for bodily decoration, and pigments were also used on papyrus. Again, pigments were ground to a fine powder with a stone tool, then mixed with a medium and applied to the desired surface. For bodily decoration, animal fats and waxes were often used as the medium. A greater variety of pigments were used by Egyptians, including:
|
,
