Suppose you’re going properly at Chemistry, however can’t ensure? On this submit, we provide you with 10 should reply Yr 11 Reactive Chemistry Follow Inquiries to get you prepared to your Yearly exams.
10 Should Reply Yr 11 Reactive Chemistry Follow Questions
Under are some Module 3 – Reactive Chemistry follow questions to arrange you to your Chemistry yearly exams. Strive the questions beneath, then see the way you went in opposition to our solutions.
Query 1 – Periodic traits
The next desk reveals the primary and second ionisation energies for Group II metals
| Steel | Atomic quantity | 1st Ionisation power (kJ mol-1) | 2nd Ionisation power (kJ mol-1) |
| Beryllium | 4 | 899 | 1757 |
| Magnesium | 12 | 738 | 1450 |
| Calcium | 20 | 590 | 1145 |
| Strontium | 38 | 549 | 1064 |
(a) Outline ionisation power.
(b) Graph ionisation power in opposition to atomic quantity for each the primary ionisation power and the second ionisation power. Use the identical set of axes for each graphs.
(c) Clarify the development that may be noticed in each graphs.
(d) Clarify why the primary ionisation power is considerably decrease than the second ionisation power for every of the weather proven within the desk.
Query 2 – Steel reactions
A pupil reacts three unknown metals with dilute hydrochloric acid.
| Steel | Remark |
| X | Quick effervescent. Many bubbles kind on the metallic floor. |
| Y | No observable response |
| Z | Gradual effervescent. Just a few bubbles kind on the metallic floor |
(a) The scholar is informed that the three metals are calcium, zinc and platinum. Establish every of the unknown metals X, Y and Z, and supply causes to your reply.
(b) Establish the fuel produced within the response of X and Z with dilute hydrochloric acid. Present chemical equations to help your reply and recommend a check that could possibly be used to confirm the fuel produced.
(c) The scholar reacts samples of X, Y and Z with water and steam. Define the seemingly merchandise and supply causes to your reply.
Query 3 – Ionic equations
When a 5.0 g pattern of magnesium metallic is positioned in a beaker with sulfuric acid, the mass of the magnesium metallic decreases by 1.7 g.
(a) Write a balanced chemical equation for the response.
(b) Write a full ionic equation and a internet ionic equation for this response.
(c) By contemplating the mass of magnesium that reacted, calculate the amount of fuel produced if the response came about at 25 oC and 100 kPa.
Query 4 – Mole questions – share composition, empirical formulation, molecular formulation
A pupil carried out an experiment during which 15.00 g of magnesium was burned in extra oxygen. The product fashioned had a mass of 24.87 g.
(a) Calculate the mass of oxygen consumed on this response.
(b) Calculate the empirical formulation of the product.
(c) Clarify why the product has an empirical formulation however not a molecular formulation.
Query 5 – Mole questions – share composition, empirical formulation, molecular formulation
The share composition of a compound is 92.3% carbon and seven.7% hydrogen. The compound is a liquid underneath customary circumstances.
(a) Calculate the empirical formulation of this compound.
(b) If the molar mass of this compound is 78.11 g/mol, decide its molecular formulation.
(c) The compound reacts with 0.87 mol of chlorine fuel to kind chlorobenzene ((C_6H_5Cl_{(l)})) and hydrogen chloride fuel. Calculate the mass of chlorobenzene produced.
Query 6 – Limiting reactants
Ammonia is produced by combining nitrogen fuel ((N_2)) and hydrogen fuel ((H_2)) at a temperature of 450 oC and pressures of 1.0 atm. How a lot (N_2) is required to react with an extra (H_2) to supply 20.0 L of ammonia?
Query 7 – Limiting reactants
A 4.58 g pattern of calcium carbonate powder is reacted with 16 mL of 1.0 M hydrochloric acid.
(a) Establish the limiting reactant and calculate the amount of (CO_2) produced at RTP (298 Okay, 100 kPa).
(b) Calculate the mass of the reactant that continues to be unreacted.
Query 8 – Redox
For the next reactions determine the oxidant and reductant.
(a) (Zn_{(s)} + CuSO_{4(aq)} → ZnSO_{4(aq)} + Cu_{(s)})
(b) (Cl_{2(g)} + Br^{ -}_{(aq)} → 2Cl^{-}_{(aq)} + Br_{2(l)})
(c) (Ca_{(s)} + 2H_2O_{(l)} → Ca(OH)_{2(s)} + H_{2(g)})
(d) (5H_2C_2O_{4(aq)} + 2MnO^{-}_{4(aq)} + 6H^{+}_{(aq)} → 10CO_{2(g)} + 2Mn^{2+}_{(aq)} + 8H_2O_{(l)})
Query 9 – Galvanic and cell potential
An experiment was carried out utilizing two half-cells: One half-cell consisted of 1.0 M (Zn(NO_{3})_{2}) answer with a zinc electrode and the opposite half-cell consisted of a 1.0 M (Cu(NO_{3})_{2}) answer with a copper electrode. The cells have been linked utilizing a salt bridge.
(a) Draw the equipment described within the experiment and label the anode, cathode, options, path of electron circulate, and salt bridge.
(b) Write the equations for each the oxidation and discount half-cells, and supply the web ionic equation for the method.
(c) Calculate the cell potential of the galvanic cell.
(d) Describe the significance of the salt bridge.
Query 10 – Price of reactions
A pupil carried out an experiment to find out the affect of reactant focus on response fee. The investigation was carried out by reacting 0.50 g pellets of aluminium with numerous concentrations of hydrochloric acid
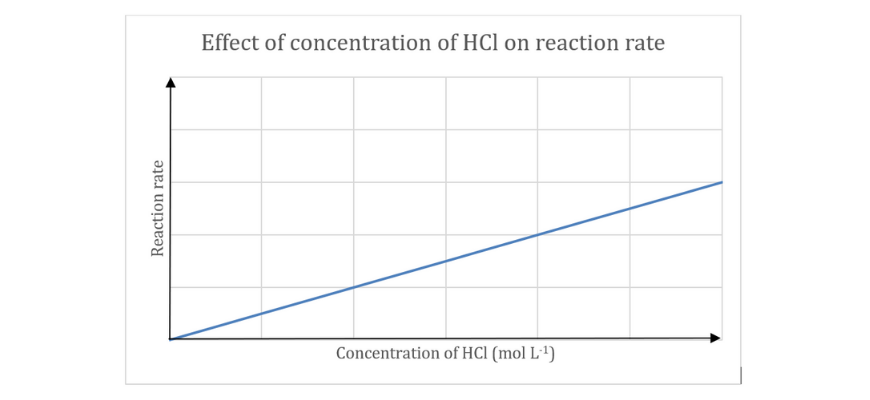
(a) Write a balanced chemical response for the above response.
(b) Establish the impartial and dependent variable on this investigation.
(c) Close to collision concept, clarify the development noticed within the graph.
(d) Draw a graph (on the identical axes) exhibiting how this graph can be shifted if the investigation was carried out at the next temperature. Give a cause to your reply.
ANSWERS
Query 1
(a) Ionisation power is the power required to take away one mole of valence electrons from one mole of gaseous atoms from the factor.
(b)
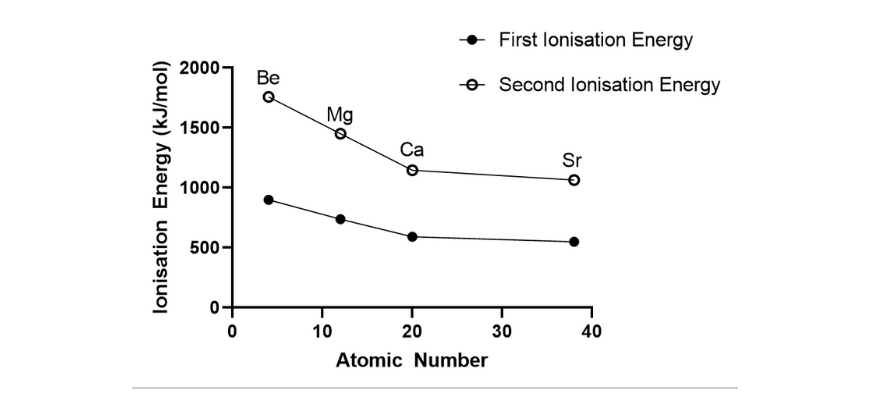
(c) From each graphs, it may be noticed that because the atomic quantity will increase down a bunch, each the primary and second ionisation power decreases. Down a bunch, new shells are added, which means that the valence electrons are additional away from the nucleus and are shielded by internal shell electrons. This ends in a decreased attraction between the nucleus and the valence electrons. Therefore, much less power is required to take away one mole of valence electrons.
(d) The primary ionisation power entails the removing of 1 mole of electrons from the valence shell of a impartial atom within the gaseous state, and ends in an ion with a 1+ cost. The second ionisation power is related to the removing of 1 mole of electrons from one mole of the ion with a +1 cost. As there are a better variety of protons than electrons within the positively charged ion, there’s a better electrostatic attraction between the valence electrons and the nucleus. Consequently, extra power is required to take away the second mole of valence electrons, resulting in the next second ionisation power.
Query 2
(a) X – calcium, Y – platinum, Z – zinc. Calcium is greater up the exercise sequence, adopted by zinc after which platinum. As essentially the most vigorous response was noticed for metallic X, it have to be calcium. As solely gradual effervescent was noticed with metallic Z, it have to be zinc. Platinum is the least reactive metallic and since metallic Y didn’t react, it have to be platinum.
(b) Each metals react with HCl to supply hydrogen fuel and a chloride salt of the metallic
(Ca_{(s)} + 2HCl_{(aq)} → CaCl_{2{(aq)}} + H_{(g)})
(Zn_{(s)} + 2HCl_{(aq)}→ ZnCl_{2(aq)} + H_{2(g)})
The manufacturing of hydrogen fuel from these reactions might be verified utilizing the pop check.
(c) As calcium is a reactive Group 2 metallic, it’s going to react intensely with water to supply calcium hydroxide and hydrogen fuel ((Ca_{(s)} + 2H_2O_{(l)} → Ca(OH)_{2(aq)} + H_{2(g)})). Zinc is much less reactive than calcium so it won’t react with water, however as a substitute will react with steam to supply zinc oxide and hydrogen fuel ((Zn_{(s)} + H_2O_{(g)} → ZnO_{(aq)} + H_{2(g)})). Platinum is very unreactive so won’t react with both water or steam.
Query 3 – Ionic equations
(a) (Mg_{(s)} + H_2SO_{4(aq)} → MgSO_{4(aq)} + H_{2(g)}).
(b) Full ionic equation: (Mg_{(s)} + 2H^{+}_{(aq)} + SO^{2-}_{4 (aq)} → Mg^{2+}_{(aq)} + SO^{2-}_{4(aq)} + H_{2(g)}).
Web ionic equation: (Mg_{(s)} + 2H^+_{(aq)} → Mg^{2+}_{(aq)} + H_{2(g)})
(c) Mass of Mg reacted (= 5.0 – 1.7 = 3.3 g).
(n(Mg) = frac{3.3}{24.31} = 0.1357 mol).
(n(Mg):n(H_2) = 1:1,) subsequently (n(H_2) = 0.1357 mol).
Utilizing the perfect fuel legislation, (PV = nRT → V = frac{nRT}{P} = frac{(0.1357 instances 8.314 instances 298)}{100} = 3.4 L) (2 sig figs). Alternatively, the equation for molar quantity (a simplification of the concept fuel legislation) can be utilized: (n = frac{V}{MV} → V = n instances MV = 0.1357 instances 24.79 = 3.4 L {(2 sig.figs)})
Query 4 – Mole questions
(a) Mass of oxygen (= 24.87 – 15.00 = 9.87 g) (2 decimal locations.) (Word: decimal locations are used to find out the variety of digits given for values decided by subtraction and addition. Learn our article on important figures for extra info)
(b) The product comprises (Mg) and (O).
(n(Mg) = frac{15.00}{24.31} = 0.617 mol).
(n(O2) = frac{9.87}{16.00} = 0.617 mol).
(n(Mg):n(O) = 1:1), subsequently the empirical formulation of the product is (MgO).
(c) (MgO) is an ionic compound which exists in a lattice construction and never as discrete molecules. Therefore, its formulation is the only ratio of its element components and never a molecular formulation.
Query 5 – Mole questions – share composition, empirical formulation, molecular formulation
(a) The empirical formulation for a compound might be simply calculated by assuming that the pattern has a mass of 100 g, which means that the odds of every factor correspond to the mass of every factor. For this query, because of this the compound would include 92.3 g of carbon and seven.7 g of hydrogen. From right here, the variety of moles of every element is calculated, and the only mole ratio decided:
(n(C) = frac{92.3}{12.01} = 7.68 mol)
(n(H) = frac{7.7}{1.008} = 7.64 mol).
(n(C): n(H) = 1:1).
Subsequently, the empirical formulation is (CH).
(b) variety of empirical formulation models ((x) = frac{textual content{molar mass }(MM)}{textual content{empirical formulation mass}} = frac{78.11}{12.01+1.008} = 6). Subsequently, the formulation is ((CH)_6 = C_6H_6)
(c) (C_6H_{6(l)} + Cl_{2(g)} → C_6H_5Cl_{(l)} + HCl_{(g)}.)
(n(C_6H_5Cl) = n(Cl_2) = 0.87 mol)
(m(C_6H_5Cl) = 0.87 instances ((6 instances 12.01) + (5 instances 1.008) + 35.45) = 97.9185 = 98 g textual content{(2 sig figs)}).
Query 6 – Limiting reactants
(N_{2(g)} + 3H_{2(g)} → 2NH_{3(g)})
Variety of moles of (NH_3):
(PV = nRT → n = frac{PV}{RT} = frac{(1.0 instances 20.0)}{(8.314 instances 723)} = 0.00332722122 mol)
(n(NH_3):n(N_2) = 2:1), subsequently (n(N_2) = frac{00332722122}{2} = 0.00166361061 mol)
(m = 0.00166361061 instances (14.01 instances 2) = 0.047 g textual content{(2 sig figs)}).
Query 7 – Limiting reactants
(a) (CaCO_{3(s)} + 2HCl_{(aq)} → CaCl_{2(aq)} + CO_{2(g)} + H_2O_{(l)}).
(n(CaCO_3) = frac{4.58}{(40.08 + 12.01 + (3 instances 16.00))} = 0.0457588 mol)
(n(HCl) = cV = 0.016 instances 1.0 = 0.016 mol)
Subsequently, the limiting reactant is (HCl).
(n(HCl): n(CO_2) = 2:1), so (n(CO_2) = 0.0080 mol).
The quantity of fuel might be discovered utilizing the perfect fuel legislation:
(PV = nRT → V = frac{nRT}{P} = (0.0080 instances 8.314 instances 298){100} = 0.19 L textual content{(2 s.f.)}).
(b) (CaCO_3) is in extra
(CaCO_3 textual content{ reacted} = 0.0080 mol)
(m(CaCO_3 textual content{ reacted}) = 0.0080 instances (40.08 + 12.01 + (3 instances 16.00)) = 0.80072 g).
Therefore, unreacted (CaCO_3 = 4.58 – 0.80072 = 3.8 g textual content{(2 s.f.)})
Query 8 – Redox
(a) (Cu^{2+}) is the oxidant, (Zn) is the reductant
(b) (Cl_2) is the oxidant, (Br^-) is the reductant
(c) (H^+) is the oxidant, (Ca) is the reductant
(d) (MnO^{-}_{4}) is the oxidant, (H_2C_2O_4) is the reductant
Query 9 – Galvanic and cell potential
(a)
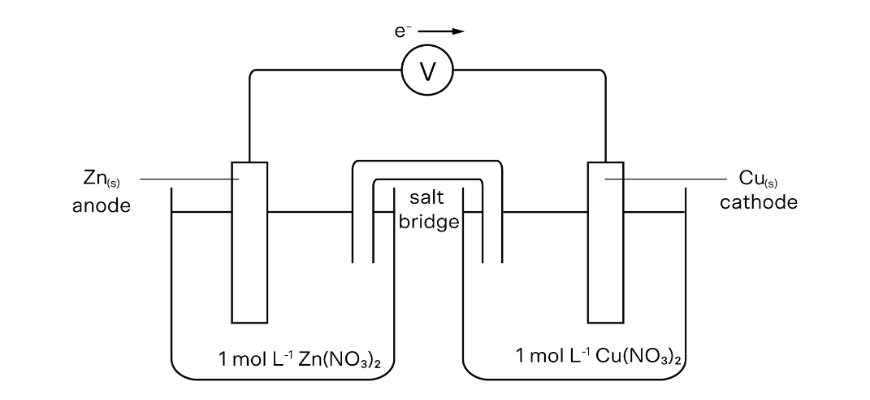
(b)
Oxidation: (Zn_{(s)} → Zn^{2+} + 2e^-)
Discount: (Cu^{2+} + 2e^- → Cu_{(s)})
Web ionic equation: (Zn_{(s)} + Cu^{2+} → Zn^{2+} + Cu_{(s)})
(c) (E_{textual content{cell}} = 0.76 + 0.34 = 1.10 V)
(d) The salt bridge completes the circuit and prevents the build-up of cost.
Query 10 – Price of reactions
(a) (2Al_{(s)} + 6HCl_{(aq)} → 2AlCl_{3(aq)} + 3H_{2(g)})
(b) The impartial variable is the focus of hydrochloric acid, and the dependent variable is the response fee.
(c) Based on collision concept, the response fee relies on the frequency of efficient collisions. An efficient collision happens when reactant molecules collide with enough power (better than or equal to the activation power) and proper orientation. On this investigation, because the focus of hydrochloric acid will increase, the probability of an efficient collision between hydrochloric acid and aluminium will increase, thereby growing the speed of response.
(d) As temperature will increase, the kinetic power of the particles additionally will increase. Consequently, reactant particles collide with the next frequency and the next common kinetic power. Thus, the variety of efficient collisions will increase, resulting in an elevated response fee.
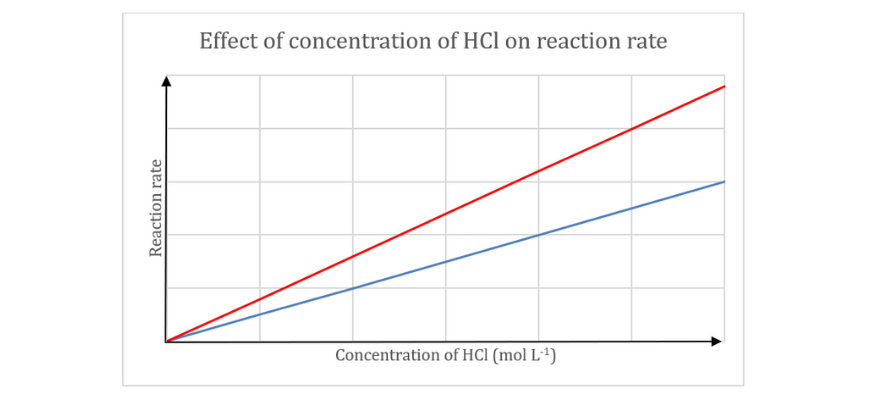 ,
,
